Abstract

BACKGROUND: Patients with persistent, isolated mild thrombocytopenia of unknown etiology (PIMT, platelet count between 100 and 149 × 109/L) are in a diagnostic and prognostic dilemma. No guidelines exist to direct workup or surveillance of these patients and there is scant data to guide prognostic discussion on the risks of development of more defined hematologic pathology, such as immune thrombocytopenia (ITP) or hematologic neoplasia. Without a comprehensive evaluation including molecular testing for acquired and inherited genetic mutations and bone marrow evaluation, diagnoses of acquired clonal disorders, hereditary thrombocytopenia syndromes, and ICUS cannot be made or ruled out, and no reliable testing exists to confirm autoimmune platelet destruction. Therefore, management may vary considerably from reassurance only to a comprehensive evaluation at the time of referral.
METHODS: We performed a retrospective cohort study to evaluate the long-term risk of developing hematologic disease (ITP or hematologic neoplasia) in patients presenting with PIMT between 1995 and 2004, to allow for extended follow-up. All data was collected via manual chart review. Subjects in the PIMT group were matched with healthy subjects with normal platelet counts in a 1:4 ratio according to age, sex, and race, with synchronization at time zero (the date of initial hematology evaluation in PIMT group) to avoid immortal time bias. Time-to-event analyses modeling risk of developing hematologic outcomes in PIMT patients and matched healthy subjects controlling for age were performed with competing-risks regression.
RESULTS:Incidence of Hematologic Disease: In the persistent isolated mild thrombocytopenia (PIMT) group, 28 (30.8%) developed hematologic disease, compared with 7 (1.9%) in the healthy subject group. This included 17 patients (18.7%) versus 1 patient (0.3%) developing ITP and 13 patients (14.3%) versus 6 patients (1.6%) developing hematologic neoplasia. Of the patients with hematologic neoplasia in the persistent isolated mild thrombocytopenia group, 8 (8.8%) developed myelodysplastic syndrome, 2 (2.2%) developed a myeloproliferative neoplasm, and 3 (3.3%) developed non-Hodgkin lymphoma; in the healthy subject group, 3 patients developed non-Hodgkin lymphoma, 1 patient developed multiple myeloma, 1 patient developed acute myeloid leukemia, and 1 patient developed CCUS.
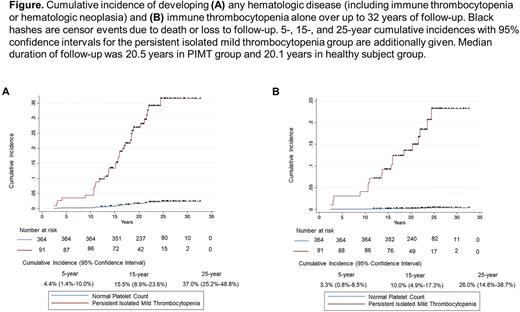
In the PIMT group, the 15-year and 25-year cumulative incidences of developing any hematologic disease were 15.5% (95% CI, 8.9%-23.6%) and 37.0% (95% CI, 25.2%-48.8%), FIGURE (A). The 15-year and 25-year cumulative incidences of developing ITP were 10.0% and 26.0%, FIGURE (B), and these incidences for developing hematologic neoplasia were 6.7% and 15.7%%. In competing-risks regression analysis controlling for age, a significantly higher risk of hematologic disease was found in the PIMT group [subhazard ratio 18.99 (95% CI 8.39-42.96), P<0.001] versus the healthy subject group.
Incidence of Bleeding and Death: Over the course of follow-up, major, clinically-relevant nonmajor, or minor bleeding events according to ISTH definitions occurred in 24 patients in the PIMT group (all in the setting of severe thrombocytopenia) versus 6 patients in the healthy subject group (all with normal platelet counts). No individuals in the healthy subject group had major bleeding, but in the PIMT group, 4 patients major bleeding events. Death occurred in 19/91 PIMT patients (21%) versus 20/364 matched healthy subjects (5.5%) despite very similar median follow-up durations.
Incidence of Systemic Autoimmune Disease: Development of non-ITP systemic autoimmune disease was more common in PIMT patients (13%) versus healthy subjects (3%). No subject in either group had a systemic autoimmune disease at baseline.
CONCLUSIONS: Controlling for age and the competing risk of death, we found a 19-fold higher risk of progression to ITP or hematologic neoplasia in patients with PIMT versus matched healthy subjects with nearly identical median follow-up duration (~20 years). During this extended follow up, PIMT patients had significantly higher rates of clinically significant bleeding, systemic autoimmunity, and death than matched healthy subjects. These findings have significant implications for initial diagnostic/workup considerations for these patients, as well as potential long-term surveillance and discussions of prognosis.
Disclosures
Grace:Novartis: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy; Agios Pharmaceuticals: Consultancy, Research Funding. Kuter:Pfizer: Consultancy; BioCryst: Consultancy, Research Funding; Momenta: Consultancy; Rubius: Current holder of stock options in a privately-held company; Cellphire: Consultancy; Novartis: Consultancy; Platelet Disorder Support Association: Consultancy; CRICO: Consultancy; Daiichi Sankyo: Consultancy; Cellularity: Consultancy; Caremark: Consultancy; Platelet Biogenesis: Consultancy; Zafgen: Consultancy; Hengrui: Consultancy; Up-To-Date: Consultancy; Shire: Consultancy; Shionogi: Consultancy; Sanofi: Consultancy; Merck Sharp & Dohme: Consultancy; Kyowa Kirin: Consultancy; Incyte: Consultancy; Genzyme: Consultancy; Dova: Consultancy; UCB: Consultancy, Research Funding; Takeda (Bioverativ): Consultancy, Research Funding; Rigel: Consultancy, Research Funding; Protalex: Consultancy, Research Funding; Principia: Consultancy, Research Funding; Kezar: Research Funding; Immunovant: Consultancy, Research Funding; BMS: Consultancy, Research Funding; argenx: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Alnylam: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Actelion (Syntimmune): Consultancy, Research Funding. Al-Samkari:Sobi: Consultancy, Research Funding; argenx: Consultancy; Rigel: Consultancy; Novartis: Consultancy; Amgen: Research Funding; Agios: Consultancy, Research Funding; Forma: Consultancy; Moderna: Consultancy; Dova: Consultancy, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal